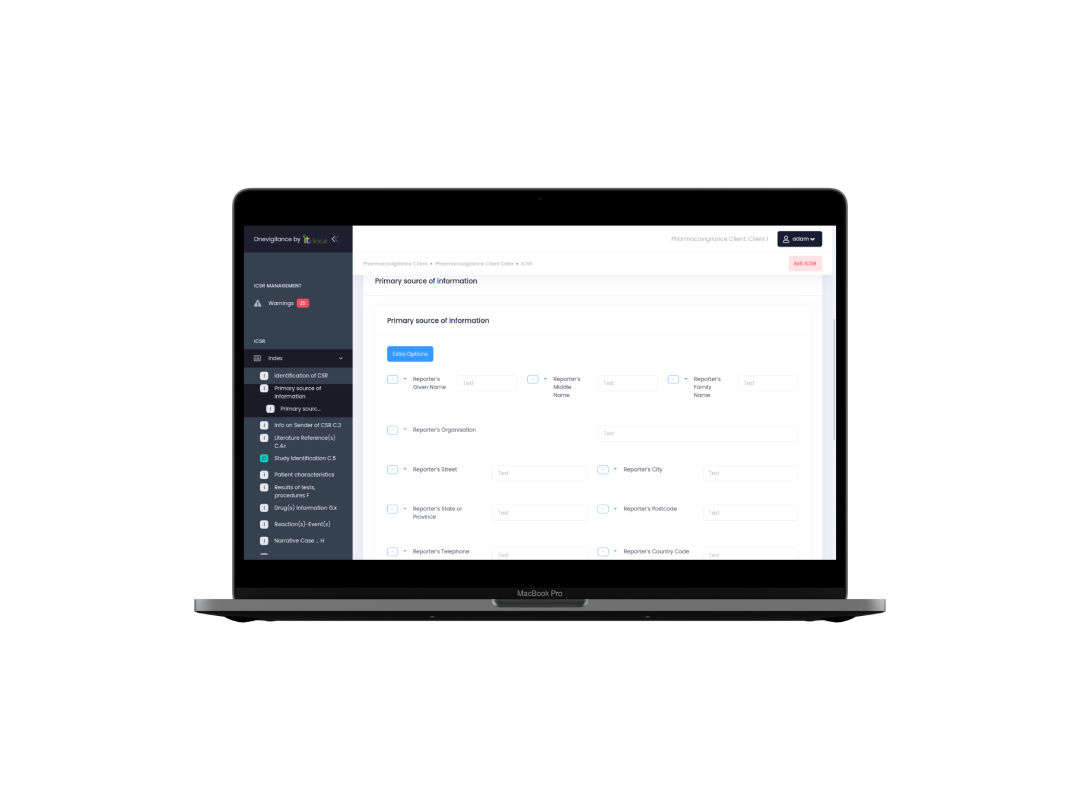
Pharmacovigilance
Centralizes the major pharmacovigilance aspects, such as Adverse Reactions from post-marketing products and clinical trials, Periodic Safety Update Reports, Licensing Agreements and Medicinal Products database. Designed for both MAHs and for CROs who work for multiple clients and therefore need data separation between MAHs.
PharmaVigilance
Centralizing major pharmacovigilance aspects, such as Adverse Reactions from post-marketing products and clinical trials, Periodic Safety Update Reports, Licensing Agreements.
Read MoreCentral hub for Pharmacovigilance
Collect, analyze and report your safety data.
Available for MAH and CROs
Manage ICSRs as a Marketing Authorization Holder (MAH) or as a CRO working on behalf of multiple MAHs.
Export and report
Export your data in various formats such as CIOMS or E2B XML.

Fully customizable
Whether your organization requires capturing additional case information or has a custom case management workflow, PharmaVigilance can adapt to your needs.
Local and Global Medical Literature Monitoring
Bibliovigilance centralizes your weekly literature searches. Thousands of publications in virtually every country to fulfill your local and global search needs.

Use a compliant software to ease your mind
E2B R3 compliant. Validated software. Support for validating your configuration. Focus on the science.

