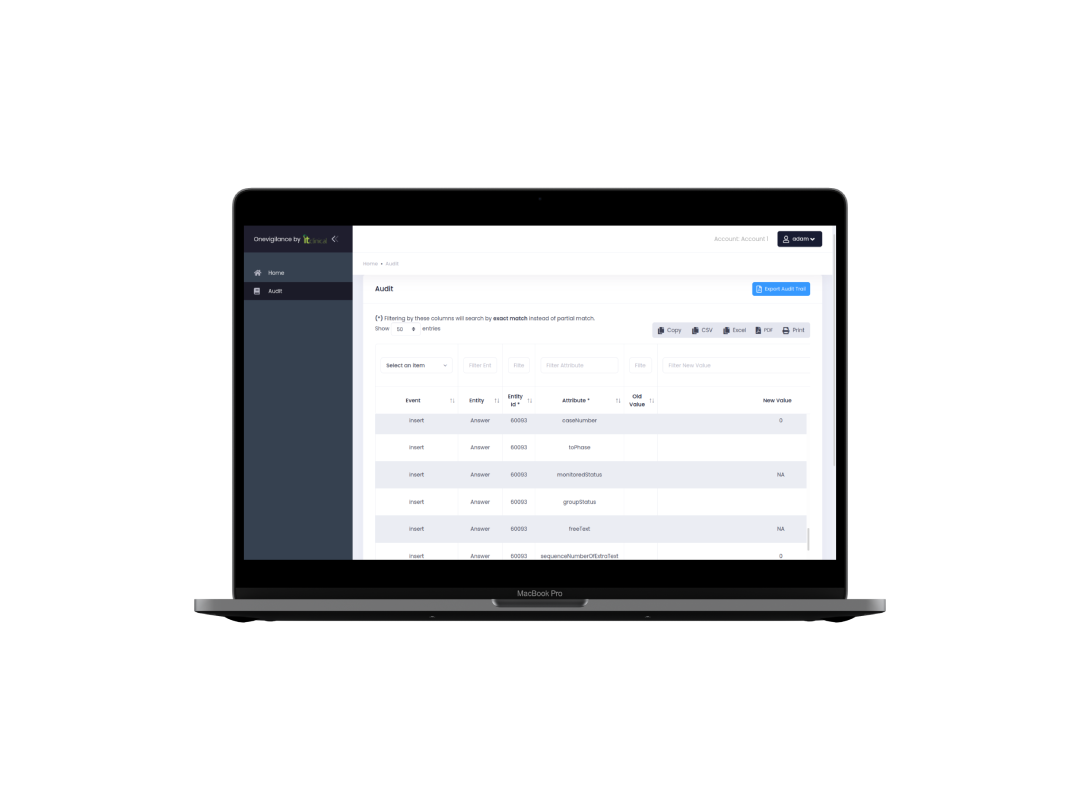
Clinical Trials
Simple and intuitive. A full fledged EDC (Electronic Data Capture) and RTSM (Randomization and Trial Supply Management) solution for your clinical trials. OneVigilance is your one-stop shop for managing your clinical trial.
Stock Management
Stock Management and Dispensing for your trial. Track, re-supply and have full drug accountability of trial supplies.
Read MoreRandomization
IRT/IWRS allows subjects to be enrolled in your clinical trial 24/7. Secure Emergency Unblinding at the tip of your fingers.
Read MoreRegulatory Compliance
By complying with regulatory requirements for electronic systems, it replaces paper based records. Compliant with industry standards, incluing the ones defined by FDA and EMA.
Read MoreStock Management
Trial Logistics Made simple
Integrated RTSM to optimize clinical trial supplies throughout the trial's lifecycle.
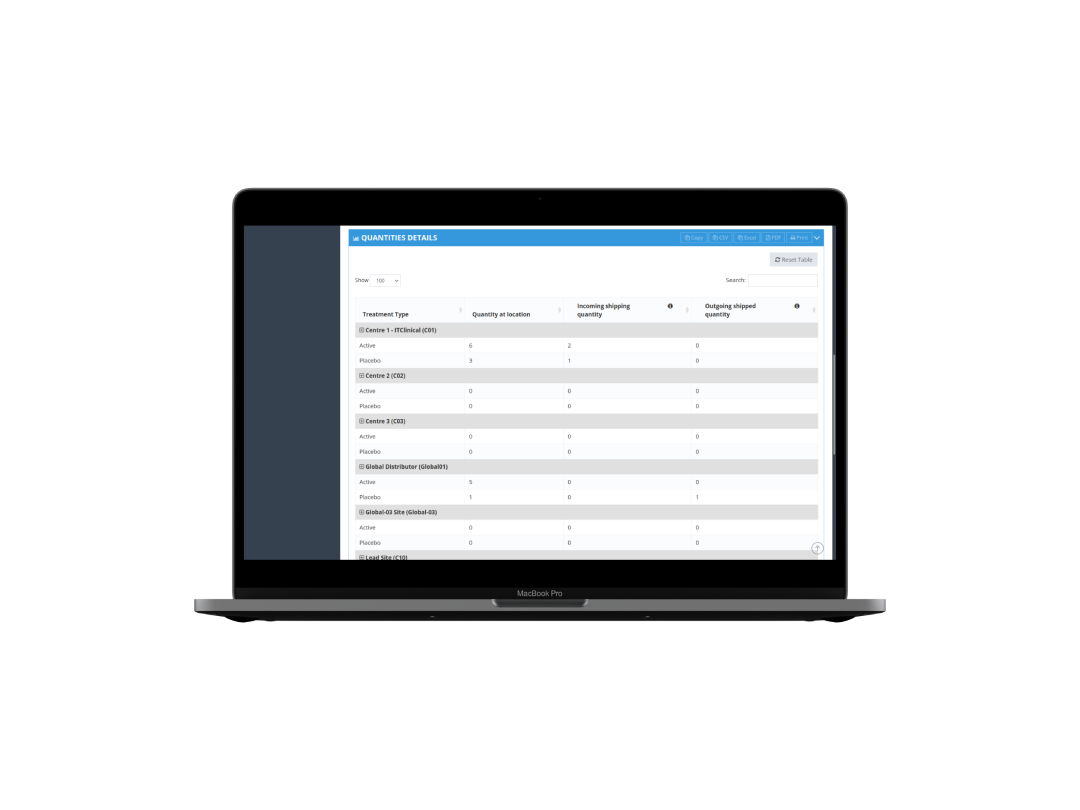

Drug dispensing
Flexible dispensing schemes for any protocol requirement.
Advanced re-supply algorithms
Choose from simple re-supply logic to advanced algorithm that takes the visit scheduling into consideration to determine supplies' consumption.
Complete life-cycle tracking
Track the entire supply chain, from manufacturer to the site and back to destruction facility.
Informative Dashboards
Blinded and unblinded views of the stock supplies to improve medication/medical device traceability.
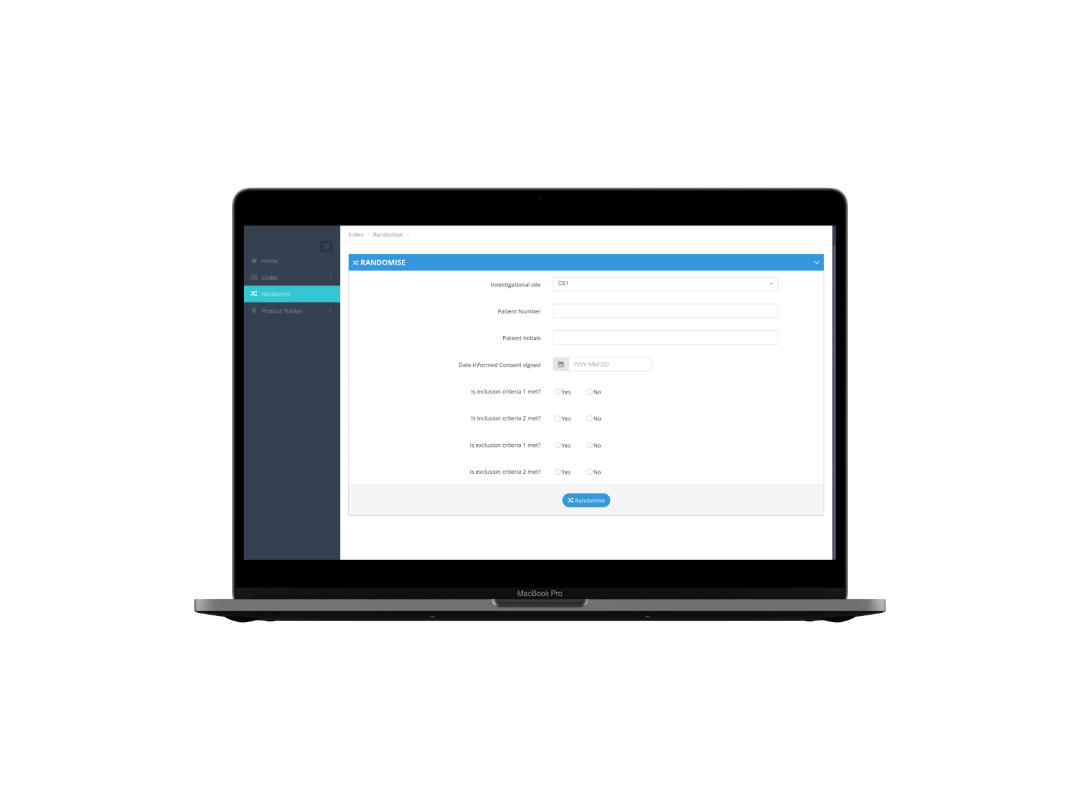
Randomization
Simple and intuitive IWRS
Enroll subjects in your clinical trial 24/7. No matter your trial's complexity, the IWRS can handle it. Anytime, anywhere.

Standalone or integrate with other systems
The randomization module can be used as a standalone or integrated into the eCRF Module. If you are already using another eCRF solution, our software can communicate with tools from other vendors.
Multiple randomization algorithms supported
No matter what you trial design is, we can handle it: Central, site, stratified, Minimization (Pocock-Simon) randomization algorithms are supported. Do you have a custom algorithm? We can design it.

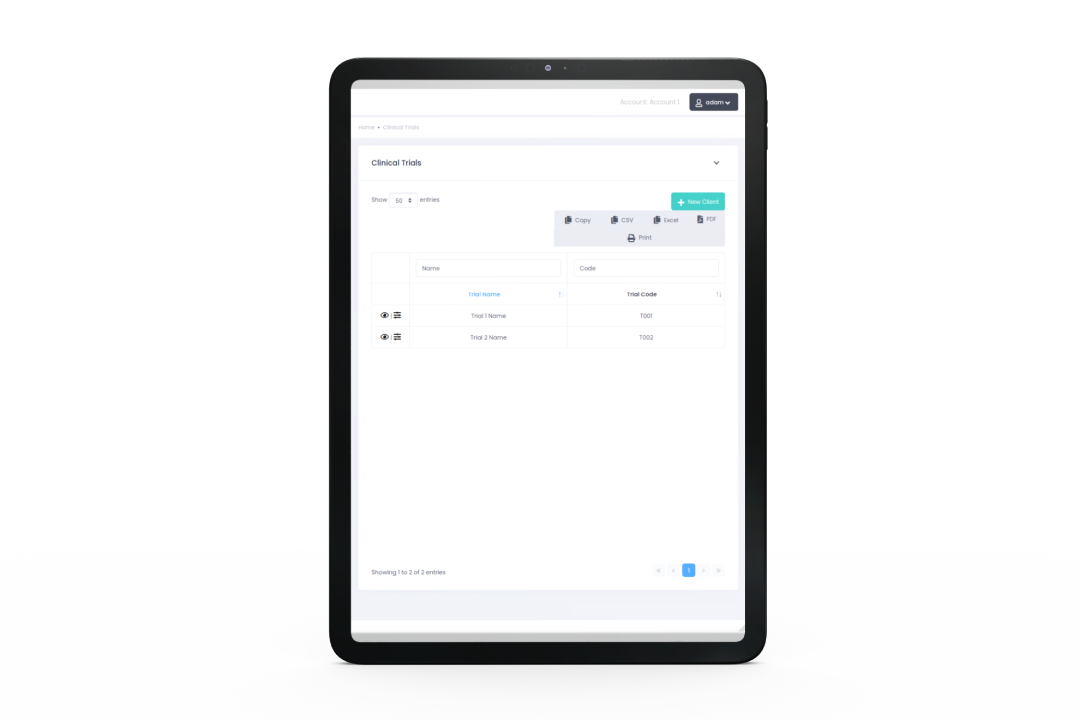
eCRF
Unified Data Access
Collect and manage subject data and export it (including in PDF format for trial master file and trial report).

Simplicity for the Investigator
Straightforward data entry tools to focus your attention on what really matters.
Tools for the Monitor and Data Manager
Ensure data quality by reviewing data entry and issuing monitoring and data management queries.


Power for the Trial Designer
eCRF design limited only by your imagination. Edit Checks to show, hide, answer, warn and email users. Data quality starts here.
Regulatory Compliance
Compliance with Industry Standards
Full compliance with unbeatable flexibility in authorization and role assignment.